Prenatal Screening Software B·R·A·H·M·S Fast Screen pre I plus
Modern, powerful and robust clinical software for prenatal screening in first and second trimester
B·R·A·H·M·S Fast Screen pre I plus is a CE marked prenatal screening software designed to ensure convenience of data entry, risk calculation and reporting for laboratories with both low and high data throughput. It is designed to be used with B·R·A·H·M·S KRYPTOR instruments and assays.
- 1st trimester trisomy and pre-eclampsia screening algorithms from the Fetal Medicine Foundation UK Note-1
- 2nd trimester risk calculation for trisomies 21 and 18 and NTD (Neural Tube Defect)
- CE marked software
- Connectivity to LIS (Laboratory Information System) and KRYPTOR instruments
First trimester trisomy and pre-eclampsia screening algorithms
B·R·A·H·M·S Fast Screen pre I plus comprises first trimester algorithms that are based on the Fetal Medicine Foundation UK (FMF) data and are updated with recently published correction factors.
| Trisomies 21, 18 and 13 | Pre-eclampsiaNote-2 | |
| Time range |
|
|
| Biomarkers | ||
| Physical markers |
|
|
The medians of the B·R·A·H·M·S biochemical markers free βhCG, PAPP-A and PlGF which are included in our software are extremely stable. These medians were established on over 220.000 healthy pregnanciesNote-3 which ensures reproductibility and robustness of the risk calculation. Therefore, a recalculation of the medians and MoMs over time is not required.
The FMF first trimester combined test performances for fetal trisomies areNote-4:
| Risk cut-off | Detection rate | False positive rate | |
| Trisomy 21 (Down's syndrome) |
1:100 |
92.1% |
4.6% |
| Trisomy 18 (Edwards' syndrome) |
1:50 |
94.6% |
2.7% |
| Trisomy 13 (Patau's syndrome) |
1:50 |
91.1% |
2.7% |
The FMF pre-eclampsia algorithm performances areNote-5:
|
Early onset |
Intermediate onset |
Late onset |
|
| Detection rate |
100% |
75% |
43% |
| False positive rate |
10% |
10% |
10% |
Second trimester trisomy and neural tube defect (NTD) screening
The B·R·A·H·M·S Fast Screen pre I plus second trimester algorithm allows calculation of risk for trisomies 21 and 18 with double test (AFP in combination with free βhCG or total hCG) between 14+0 and 19+6.
Between 15+0 and 19+6, the AFP concentration can be used as a marker of fetal neural tube defect (NTD).
B·R·A·H·M·S Fast Screen pre I plus: More than just a risk calculation tool
 Simple and user-friendly interface
Simple and user-friendly interface
- Find here a quick guided tour through B·R·A·H·M·S Fast Screen pre I plus interface.
- B·R·A·H·M·S Fast Screen pre I plus is available in 6 languages (English, German, French, Spanish, Italian and Turkish).
 Maximum data security and traceability
Maximum data security and traceability
- Software audit option for external quality control
- Internal audit trails to trace all actions
- Pop-up warnings if data are missing or out of range
- Validation of data before production of report
- All data are saved automatically
- Four levels of user credentials for data entry and management
 Effective management tools
Effective management tools
- Statistic and audit reports
- Pregnancy outcome requests and follow-up information
- Database search tool
- Export data for statistics and research
- Printing reports one by one or in defined batches
- Printing, saving and sending reports automatically by email
- Possibility to keep extended patient data for record purposes
 High connectivity
High connectivity
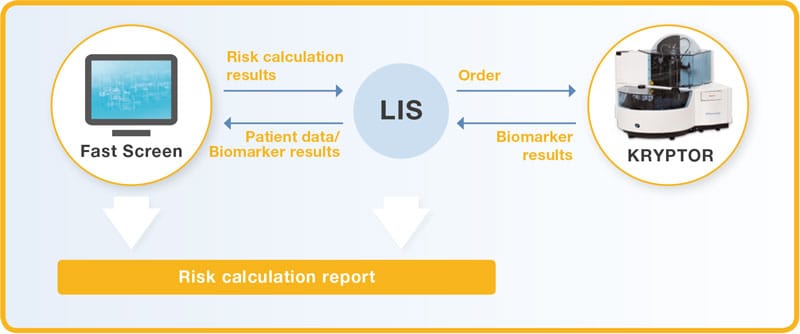
- Manual entry of patient data and connection to KRYPTOR

- Automatic data transfer via Laboratory Information System (LIS)
Further links on prenatal screening products and assays
- Download our brochure about B·R·A·H·M·S Fast Screen pre I plus.
- Download our literature review about prenatal screening software algorithms.
- Find here a quick guided tour through B·R·A·H·M·S Fast Screen pre I plus interface.
- Find here all our prenatal screening brochures, product sheets and other informational material.
- A 60 days free trial of the software is possible, please contact us for more details.
Notes and references
Note-1: Trisomy risk assessment in the 1st trimester is also possible using the algorithm developed by the Fetal Medicine Foundation Germany.
Note-2: For pre-eclampsia screening, any combination of markers is possible, best performances are reached when the 4 markers are measured (i.e. PAPP-A, PlGF, MAP and UAPI)
Note-3:
Concerning free βhCG and PAPP-A medians please refer to D. Wright et al., “First-trimester combined screening for trisomy 21 at 7-14 weeks’ gestation” Ultrasound Obstet. Gynecol., vol. 36, no. 4, pp. 404–411, 2010.
Concerning PlGF medians please refer to P. Pandya et al., “Maternal serum placental growth factor in prospective screening for aneuploidies at 8-13 weeks’ gestation” Fetal Diagn. Ther., vol. 31, no. 2, pp. 87–93, 2012.
Note-4: M. Santorum, D. Wright, A. Syngelaki, N. Karagioti, and K. H. Nicolaides, “Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13” Ultrasound Obstet. Gynecol., vol. 49, no. 6, pp. 714–720, Jun. 2017.
Note-5: N. O’Gorman et al., “Accuracy of competing risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation” Ultrasound Obstet. Gynecol., vol. 208, no. 2, pp. 109–112, Jan. 2017.