Pre-eclampsia: First Trimester Screening
Pre-eclampsia – a life-threatening pregnancy related disorder
- Pre-eclampsia is a life-threatening pregnancy related disorder which affects about 2-8% of pregnanciesRef-1
- New onset of hypertension and/or proteinuria after 20 weeks of gestation are the first clinical signs and symptomsRef-1
- About 20% of the affected pregnancies are complicated by additional symptoms such as abdominal pain, haemorrhage, placental abruption or severe HELLP-syndrome (Hemolysis, Elevated Liver enzymes, Low Platelets)Ref-2
- Eclampsia is the final stage of the disease, associated with severe tonic-clonic seizures and deathRef-1-2
Pre-eclampsia screening strategy
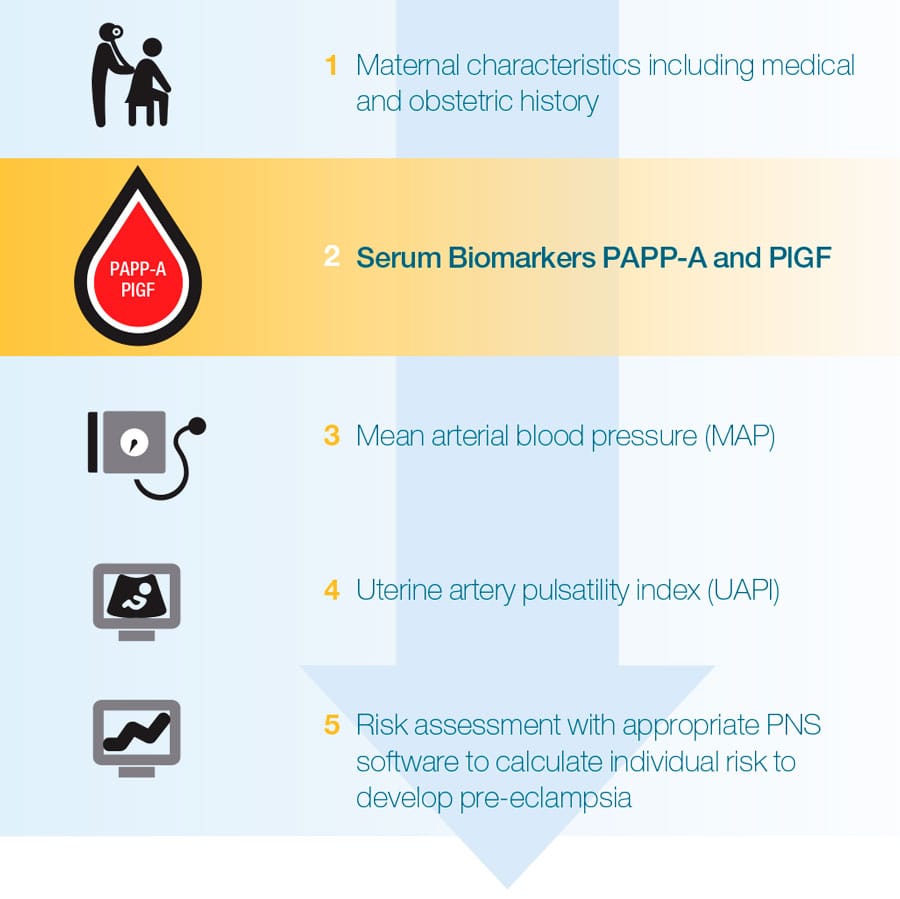
First trimester screening for pre-eclampsia is performed in weeks 11+0-13+6 of gestation and combines maternal characteristics and history with findings of biophysical and biochemical tests including the measurement of serum biomarkers PAPP-A and PlGF.
The result of the screening is calculated with a risk calculation software and is provided as the individual risk of a pregnant woman to develop later in pregnancyRef-3.
Screening performance
Using the traditional screening method, based on maternal history only, detection rate for women who are at risk for developing pre-eclampsia is about 30%Ref-4.
Screening performance is significantly improved when maternal characteristics are combined with PlGF, PAPP-A, mean arterial pressure (MAP) and uterine artery Doppler, resulting in a detection rate of >90% for a false positive rate of 5% for pre-eclampsia before 34 weeksRef-5-6.
Pre-eclampsia screening can be easily integrated into clinical routine pregnancy assessments in first trimester and should be provided to every pregnant womanRef-7.
The high sensitivity assays Thermo Scientific B·R·A·H·M·S PlGF plus KRYPTOR and B·R·A·H·M·S PAPP-A KRYPTOR can reliably detect PlGF and PAPP-A in maternal serum already at weeks 11–13+6 of gestation to support a high quality first trimester pre-eclampsia screening.Ref-3-6
Prevention with low dose aspirin
A meta-analysis showed that the use of aspirin (150 mg/day) can reduce the incidence of pre-eclampsia by about 50% if started before 16 weeks of gestation in high risk patientsRef-8.
A recent double-blinded, placebo-controlled multi-center study (ASPRE trial) confirmed the beneficial effects of aspirin. It was shown that aspirin at a daily dose of 150 mg given from week 12 to week 36 could reduce the rate of:
- Pre-eclampsia onset before 37 weeks by 62%
- Pre-eclampsia onset before 34 weeks by 82%
in women identified at high risk for preterm pre-eclampsia by the first trimester combined screeningRef-9.
Benefits of early pre-eclampsia screening (weeks 11-13+6)
- Early identification of high risk pregnancies for pre-eclampsia before first clinical symptoms appear
- Early risk assessment allows for closer surveillance and timely administration of aspirin (<16 weeks) to significantly reduce the incidence of pre-eclampsia
Further links on prenatal screening products and assays
- Download our brochure on first trimester pre-eclampsia screening.
- Download our literature review on first trimester pre-eclampsia screening.
- Download our flyer on prevention and prediction of pre-eclampsia.
- Download our fact sheet on pre-eclampsia screening.
- Find here a 10 minutes animation about management throughout pregnancy.
- Find here all our prenatal screening brochures, product sheets and other informational material.
Other useful links
- Fetal Medicine Foundation
- European Foundation for the Care of Newborn Infants (EFCNI)
- PREECLAMPSIA Foundation
 Join the pre-eclampsia awareness campaign from the European Foundation for the Care of Newborn Infants (EFCNI).
Join the pre-eclampsia awareness campaign from the European Foundation for the Care of Newborn Infants (EFCNI).
World Pre-eclampsia Day: act early! screen early! >
References
Ref-1: L. Ghulmiyyah and B. Sibai, “Maternal Mortality From Preeclampsia/Eclampsia” Semin. Perinatol., vol. 36, no. 1, pp. 56–59, 2012.
Ref-2: C. E. Powe, R. J. Levine, and S. A. Karumanchi, “Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease” Circulation, vol. 123, no. 24, pp. 2856–2869, 2011.
Ref-3: R. Akolekar, A. Syngelaki, R. Sarquis, M. Zvanca, and K. H. Nicolaides, “Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks.” Prenat. Diagn., vol. 31, no. 1, pp. 66–74, Jan. 2011.
Ref-4: L. C. Poon et al., “ASPRE trial: incidence of preterm preeclampsia in patients fulfilling ACOG and NICE criteria according to risk by the FMF algorithm.” Ultrasound Obstet. Gynecol., vol. 44, no. 0, pp. 1–43, Jan. 2018.
Ref-5: N. O’Gorman et al., “Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation.” Am. J. Obstet. Gynecol., vol. 214, no. 1, p. 103.e1-103.e12, Jan. 2016.
Ref-6: L. C. Poon and K. H. Nicolaides, “First-trimester maternal factors and biomarker screening for preeclampsia” Prenat. Diagn., vol. 34, no. 7, pp. 618–627, 2014.
Ref-7: D. L. Rolnik et al., “Early screening and prevention of preterm pre-eclampsia with aspirin: time for clinical implementation.” Ultrasound Obstet. Gynecol., vol. 50, no. 5, pp. 551–556, 2017.
Ref-8: P. Bujold, Emmanuel MD, MSc, Roberge, Stéphanie, MSc, Yves Lacasse, MD, MSc, Marc Bureau, MD, Franc¸oisAudibert, MD, MSc, Sylvie Marcoux, MD, PhD, Jean-Claude Forest, MD, PhD, and Yves Giguere, MD, “Prevention of Preeclampsia and Intrauterine Growth Restriction With Aspirin Started in Early Pregnancy” Obstet. Gynecol., vol. 116, no. 2, pp. 402–414, 2010.
Ref-9: D. L. Rolnik et al., “Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia.” N. Engl. J. Med., vol. 377, no. 7, pp. 613–622, Jun. 2017.